REJEON PCL Filler Injection Anti-wrinkle lifting and firming
The Origin of REJEON PCL
In the past 20 years, our understanding of one of the most complex areas of the human body – the face – has improved dramatically, with several new anatomical structures having been identified.
At the same time, a plethora of non-surgical
procedures have become available for treating
the signs of ageing and restoring the youthful
appearance of the face. REJEON is the first, and
currently the only, collagen sti mulator that is made of polycaprolactone microspheres, which contribu te to its durable aesthetic enhancements. REJEON
‘s unique properties mean it is a desirable op tion for a range of soft-tissue procedures.

Summary
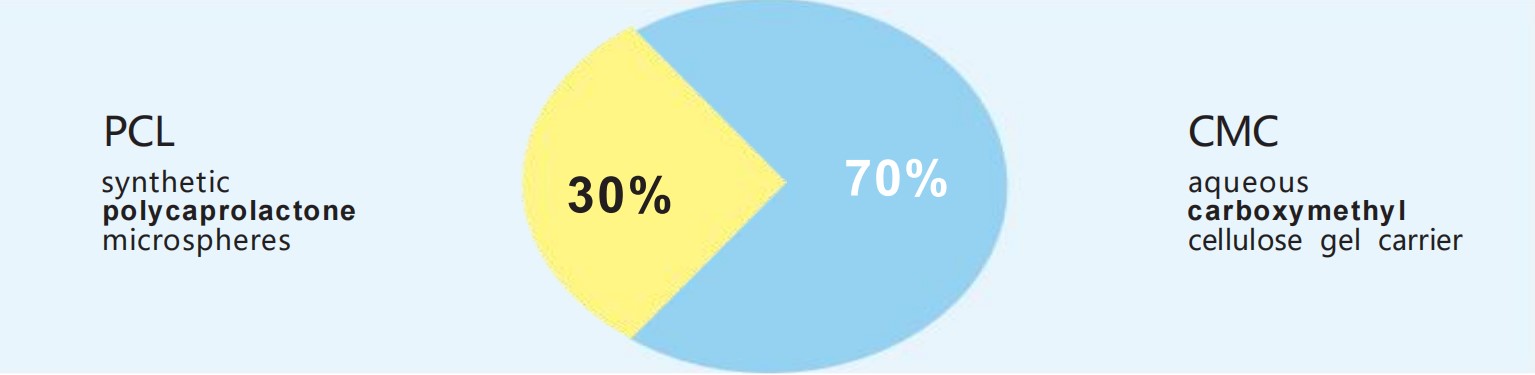
The composition of R E J EO N ,
7 0 % aqueous CMC- based
gel carrier and 3 0 % PCL
composition, allows for
an immediate filling effect
caused by CMC, followed by stimulation of the body’ s own collagen ( neocollagenesis) .
CMC is resorbed 2 to 3
months post- injection and is progressively replaced by the patient’ s own
collagen ( predominantly type I) stimulated by
PCL microspheres. The microspheres of PCL are also bioresorbable.
REJEON has a number of attributes that make it an attractive option as a dermal filler:
①Encapsulation of polymer microspheres, within approximately 1 month, and the associated collagen scaffold prevent further inflammatory reactions from occurring13
②The enduring collagen type in the injected site is predominantly the‘ mature’ collagen scaffold of collagen type I5
a) The reduction of collagen type III means no further stimulation of the inflammatory response
③The degradation of REJEON constituents is completed by hydrolysis, leaving just water and carbon dioxide
④Because the final volume within the treated area is greater than the volume of Ellansé injected, there is no requirement to ‘touch up’the treatment
a) The final volume is greater than the volume injected by 20–30% due to formation of collagen type I fibres11
⑤The availability of two versions of REJEON with different durations of action means that the length of treatment effect can be tailored to a patient’s
requirements
a) This is achieved by varying the length of the PCL chains, allowing for predictable, controlled and adjustable bioresorption
⑥The treatment technique is the same regardless of the R E J E O N product selected a) Same:
● Rheological properties
● Technique
● Syringe
● Needle/cannula
REJEON PCL Unique composition
REJEON PCL is composed of a unique, patented
blend of:
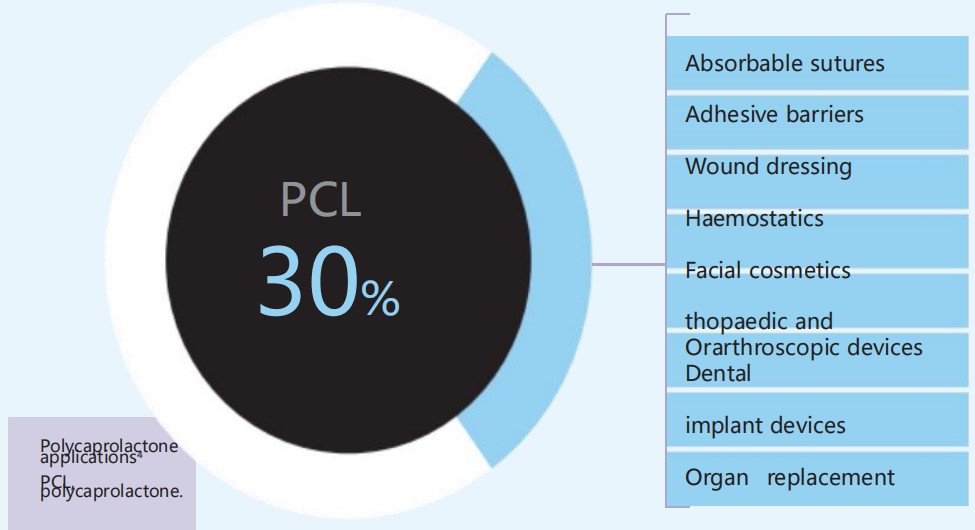
● 7 0 % carboxymethyl cellulose ( CMC) - based gel carrier
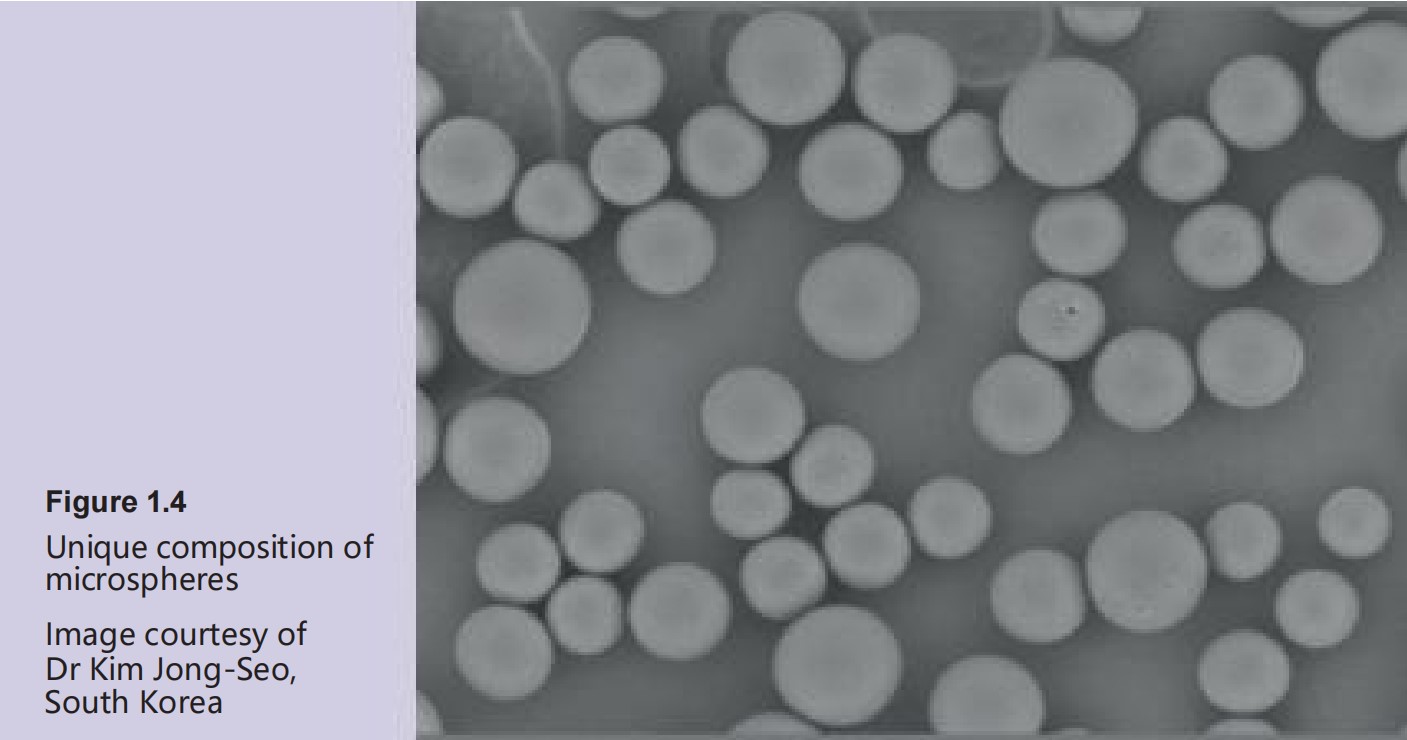
● 3 0 % polycaprolactone ( PCL) microspheres ( Figure 1 .4 ) 3 , 4 , 5
The PCL microspheres are held in
homogeneous suspension in the CMC-based gel carrier. PCL and CMC both have an excellent and proven biocompatibility profile.
REJEON PCL raw materials come from Gemany
PCL MICROSPHERES
PCL is a non-toxic medical polyester, first synthesised in the early 1930s4, that is
attractive for use in dermal fillers because of its ease of bioresorption; it is naturally hydrolysed into carbon dioxide and water within the body5.
The PCL microspheres used in
RE JEON are designed to offer
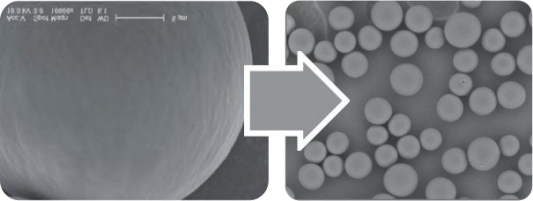
optimal biocompatibility6 . They have a smooth surface, a
spherical shape and a size of
approximately 25–50 μm
PCL has an excellent safety profile3 and has been used in the biomedical field for more than 70 years for a range of applications, from sutures to tissue and organ replacements by 3D printing (Figure 1.6)4. It is also used in CE-marked and US Food and
Drug Administration (FDA)- approved products.
PROPERTIES OF CMC
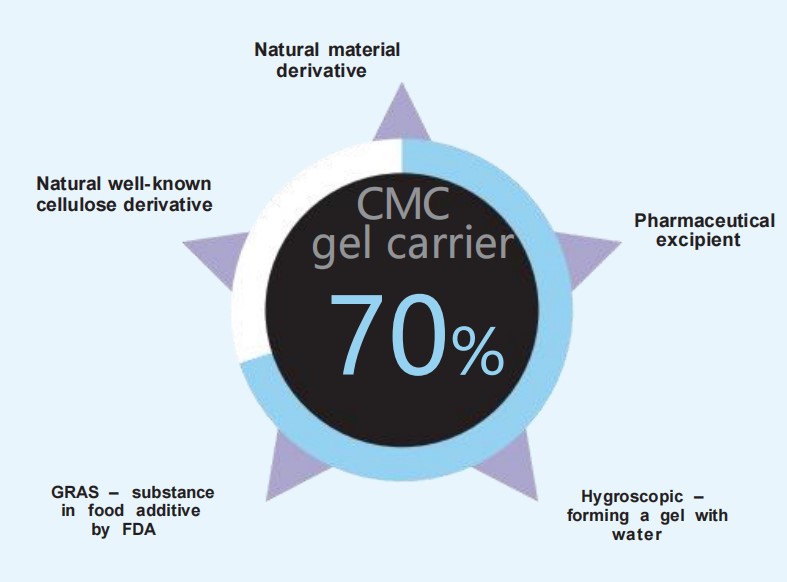
CMC is a natural material derived from cellulose; it is not cross-linked, and is non- toxic. Its other properties include (Figure 1.7)4 :
● It is a recognised pharmaceutical excipient
● It is hygroscopic
● It has been designated by the FDA as generally recognised as safe ( GRAS)
● Resorption occurs in 2 – 3 months
The core advantages of REJEON PCL Filler
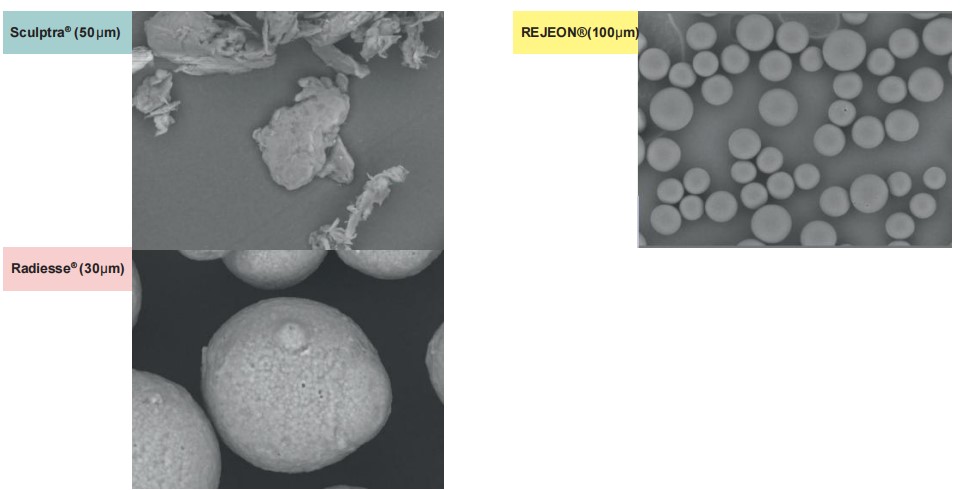
REJEON PCL has a unique and perfect microsphere, with a particle size that meets international safety standards and a smooth surface that can continuously promote the growth of collagen.
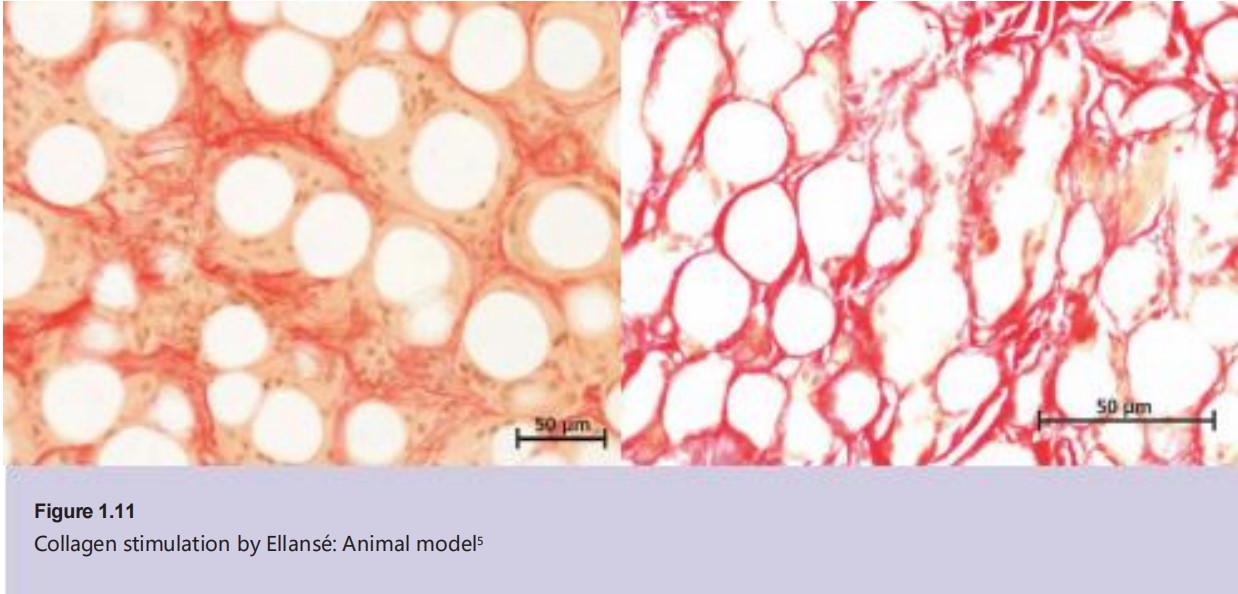
Collagen stimulation by REJEON:Scientific evidence
REJEON has been
tested in an animal
model where rabbits were injected with
either REJEON S
(PCL-1) or REJEON M (PCL-2) to investigate neocollagenesis5 .
Nine months after injection of PCL- 1 ,
neocollagenesis had occurred and the PCL microspheres of PCL- 1 had been completely resorbed ( Figure 1 . 1 1) 5 .
Meanwhile, with PCL- 2 at 9 months,
there was evidence of formation of
type I and type III collagen around
PCL microspheres. At 2 1 months post- injection, PCL- 2 microspheres were still present in the injected tissue5 .
In a pilot study of RE JEO N in humans, patients were enrolled to receive Ellansé injected intradermally into the temple
region9 . Histological analysis of tissue obtained from the biopsies revealed
collagen formation around the injected PCL particles ( Figure 1 . 12 ) 9 , supporting similar findings previously shown in
rabbit tissue5 .
REJEON mechanism of action
REJEON has two distinct phases of activity (Figure 1.9)1,4 :
● Step 1 : Immediately after injection, the CMC component provides temporary volume,
which gradually decreases over 2–3 months
● Step 2 : The PCL microspheres induce
neocollagenesis of types I and III collagen, with more persistent type I collagen
structure gradually increasing over 1 – 3 months and the PCL microspheres
becoming embedded in the type I collagen
scaffold. The resulting collagen volume
replaces the initial volume increase caused by the CMC gel
The collagen scaffold stimulated by the PCL
microspheres persists after they have been resorbed, leading to the durable volume increase seen with REJEON
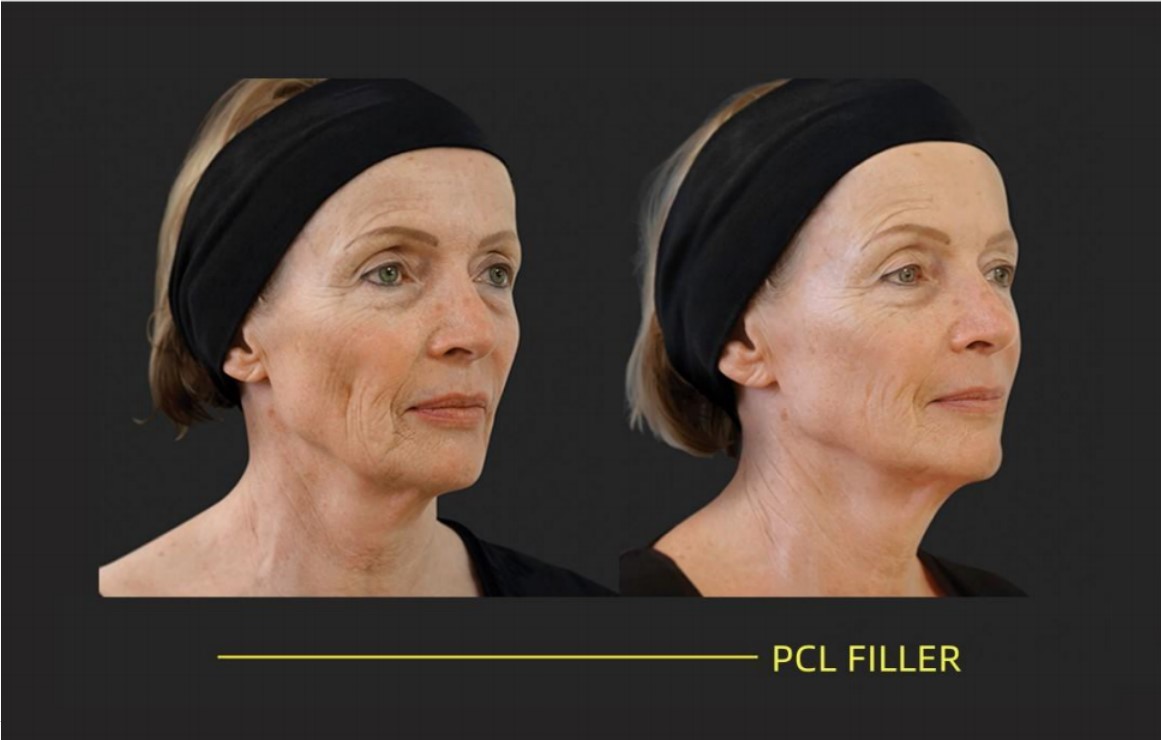
REJEON PCL Filler has good results
REJEON PCL Filler is a high-end long-lasting filling agent that can smooth out the traces left by time and restore a plump and youthful appearance to the face.
REJEON PCL FILLER CUSTOMER FEEDBACK

We intend to share our expertise and
knowledge in when and how to incorporate r ej e o n in clinical practice. I hope it will benefit the reader the same way it has worked for me for the past 10 years: offering safer treatments with a better outcome and long-lasting results. RE JEON is a fundamental tool in my practice and has made me a better injector! ”
Dr Francisco de Melo
Plastic Surgeon, UAE

“ RE JEON has been my favourite dermal filler for
7 years. This book will help you to master the use of
RE JEON and you will fall in love with it. ”
Dr Shang- Li Lin
Dermatologist, Taiwan

“ The improvement in structure and skin
quality resulting from RE JOE N ’ s unique
neocollagenesis is unmatched. Undoubtedly one of the best tools for clinics that want maximum efficacy and safety in an injectable product. R E J EO N has the
capacity to provide long- lasting lifting and enhanced facial structure with just a single session. ”
Dr Ingrid Ló pez- Gehrke
Dermatologist, Mexico

“ I find great pleasure in using RE JEON due to its incredible vol umi sing effect. This allows less
product to be used, and through the real production of collagen type I, has a true capacity for skin
regeneration. Many patients tell me: ' It is the first time
I have something that lasts’, or ' Look at the quality of my skin’. Definitely my favourite filler. ”
Dr Pierre Nicolau
Plastic Surgeon, Spain
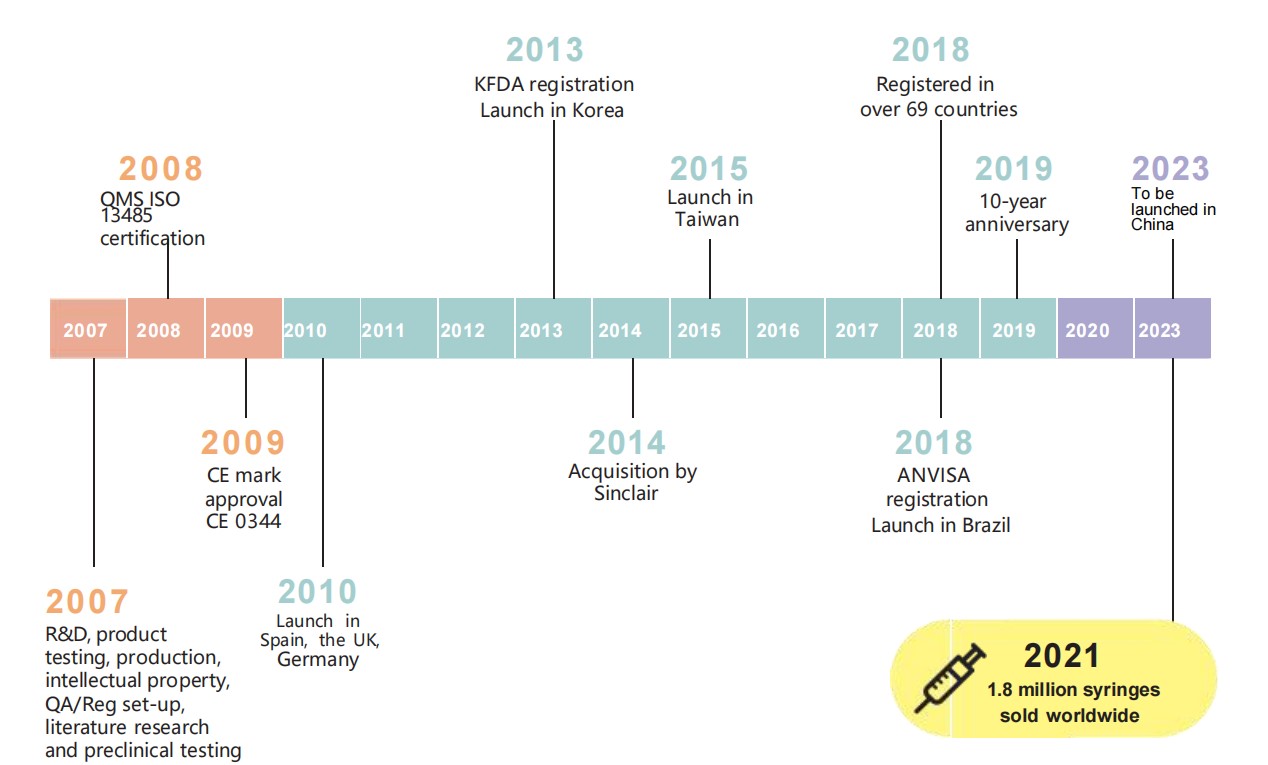
REJEON MAJOR MILESTONES
Following extensive research and development, and clinical
testing,REJEON gained ISO 13485 Quality Management System certification in
20081 (Figure 1.2). In 2009, the European Conformity (CE) mark approval was
granted, leading
to the highly successful launch of
the product in the UK, Germany and Spain. Other launches followed, with being registered in more than 69
countries by 2018. By 2019, the
10-year anniversary of rejeon, more
than 1 million syringes had been sold
worldwide. But the success story didn’t stop there, with a new manufacturing site in the Netherlands starting
production in 2020
REJEON PCL PRODUCT Detail
1ml/piece
Accept OEM customized packaging